News
Apply Electron Probe Microanalyzer (EPMA) for Analysis of High Carbon Chromium Bearing Steel
High carbon steel and steel alloys used for mechanical parts and construction are called specialty steel and are utilized widely in various fields including automobile and aircraft industries. Specialty steel is subjected to a heat treatment, which is called quenching and tempering, to refine the mechanical properties such as strength and toughness. In the case of bearing steels or tool steels that contains a high percentage of carbon, annealing is performed to obtain an intended property.
Carbon steel that contains over 0.6 % carbon (C) is classified as high carbon steel, among which bearing steel is a typical steel type. This article introduces an example analysis of high carbon chromium bearing steel (SUJ) using a field emission type electron probe microanalyzer (Shimadzu EPMA-8050G).
According to S. Yoshimi
High Carbon Chromium Bearing Steel (SUJ) Bearing steel is processed by heating pearlite steel at the eutectoid temperature for a long time. This process converts netlike cementite (Fe3C, iron carbide) into spherical lumps, which improves machinability. By adding chromium, hard spherical chromium carbide particles measuring 1 μm or less are distributed uniformly in the martensite structure. This steel is used typically for bearings. Bearing steel must have mechanical properties of high wear resistance and durability as well as outstanding rolling contact fatigue life. Spheroidizing causes the uniform distribution of spherical carbide particles, which improves mechanical properties. On the other hand, material defects such as impurities or inclusions affect the rolling contact fatigue life. In particular, B-type inclusions that align granularly in a discontinuous way and C-type inclusions (oxide-based non-metal inclusions) that disperse irregularly have a significant impact. The removal and fine scattering of such inclusions is needed.
Fig. 1 shows the internal mapping data of high carbon chromium bearing steel, which indicates that spherical chromium oxide particles of approx. 1 μm are distributed uniformly.
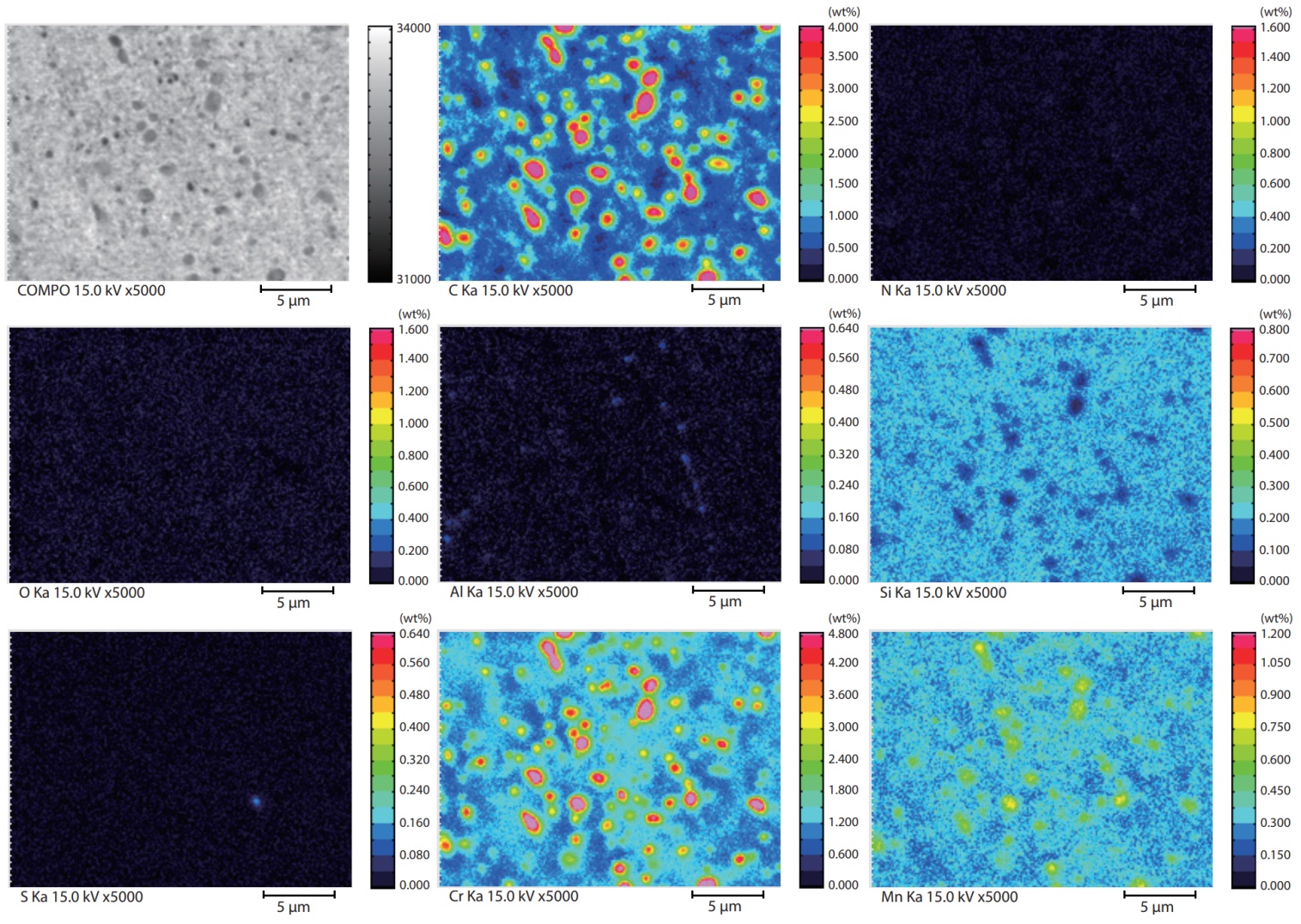
Fig 1. Mapping Analysis of High Carbon Chromium Bearing Steel
Nitriding
Nitriding is a thermochemical treatment for diffusion coating using nitrogen to produce minute nitrides on the surface of steel that contains elements having an affinity for nitrogen (N) stronger than that for iron (Fe), such as aluminum (Al), chromium (Cr) or molybdenum (Mo). This method is used as a surface hardening method to generate a hard, corrosion resistant nitrogen compound layer on the steel surface to improve wear resistance. In particular, aluminum is a key element that influences the hardness of the nitrided layer.
Fig. 2 shows the results of mapping analysis of the nitrided surface layer on high carbon chromium bearing steel (SUJ). In addition to the chromium oxide particles, a distribution of minute chromium nitride (CrN) of approx. 100 nm and aluminum nitride (AlN) of 100 nm or less are observed.
Phase Analysis and State Analysis
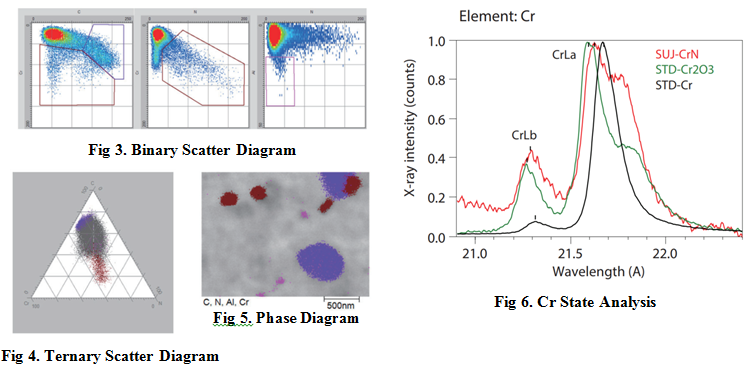
Phase analysis extracts the strength (concentration) plotted on a scatter diagram as a point set (cluster) to accurately represent each compound phase. Fig. 3 shows the results of extracting C-Cr, N-Cr, and N-Al into binary scatter diagrams respectively and Fig. 4 shows the results of extracting C-N-Cr in a ternary scatter diagram. The compounds, which are chromium carbide, chromium nitride, and aluminum nitride, are identified respectively as purple, brown, and magenta regions in Fig. 5.
Chromium can be state analyzed by using Cr-L rays. When chromium forms a compound, the ratio of Cr-Lβ/Cr-Lα changes from that of chromium only. Chromium nitride is known to have a Cr-Lα peak shape differing from that of chromium oxide, and the results are shown in Fig. 6.
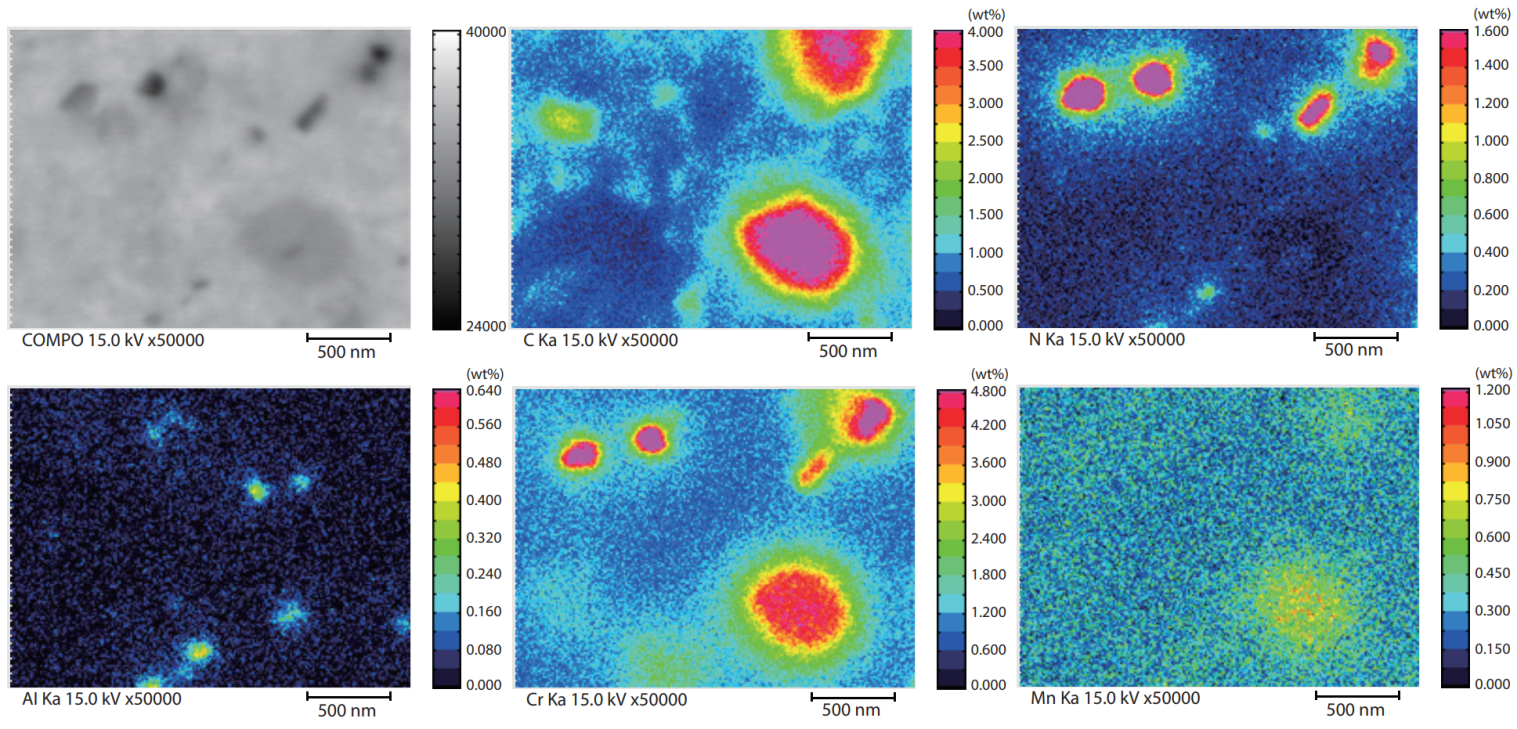
Fig 2. Mapping of a Carbonitrided Surface on High Carbon Chromium Bearing Steel
Reference: Application News - No. P101, Shimadzu Corporation
Source: ETA
Others
- TECOTEC GROUP ATTENDED SHIMADZU’S SERVICE MANAGER MEETING IN 2022
- TECOTEC HANDED OVER EDX-7000 X-RAY FLOURESCENCE SPECTROMETER AT NIDEC CHAUN CHOUNG VIETNAM
- INSTALLATION OF CHIP PROCESSING SYSTEM – LANNER/ GERMANY
- TECOTEC completed installation of EDX-LE Energy dispersive X-ray Fluorescence spectrometer at DYT Vina
- TECOTEC DELIVERED AND INSTALLED THE 2ND X-RAY FLUORESCENCE SPECTROMETER - EDX-LE PLUS AT TABUCHI
- TECOTEC Group has handed over PDA-7000 Optical Emissions Spectrometers for Nihon Plast Vietnam
- Bowman XRF Coating Measurement System For Electroless Nickel Plating
- TECOTEC DELIVERED AND INSTALLED SMX-2000 SYSTEM TO NIDEC TECHNO MOTOR VIETNAM



