News
X- Ray Fluorescence Analysis of Residual Catalysts EDX-7000
Many familiar industrial products are made of organic compounds and their manufacturing processes involve various synthesis reactions and metal catalysts. However, the residual contents of catalysts need to control strictly in products after finishing reaction. In this example, Palladi (Pb), was used as a catalyst for a cross-coupling reaction Suzuki-Miyaura.
Chemicals and equipments
- Analysis machine: EDX-7000/Shimadzu
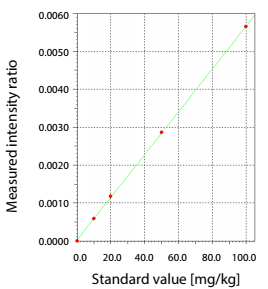
- Measurement method: Use calibration curve method of Pd, Which build from contents 1 ppm, 10 ppm, 20 ppm, 50 ppm và 100 ppm.

Preparing sample
Analysis samples are liquid, They are contained into sample container which was covered with 5 μm thick polypropylene film.
Analysis process, Image
The amount of Pd was quantitatively analyzed by the calibration curve method at three stages: Prior to adding the catalyst, after adding the catalyst, and after separating and removing the catalyst.
The analysis process flow, images of the samples at each stage in analysis, and the X-ray fluorescence (XRF) spectra are shown in Fig.
- Step 1: Reagents other than the catalyst of sample 1 is mixed for eacohf Fluorophore 1 and 2.
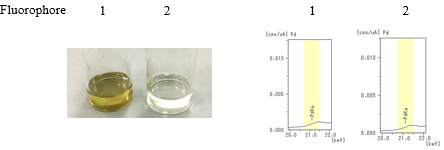
- Step 2: The catalyst (palladium acetate) was added to each solution.
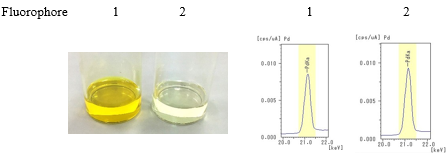
- Step 3: After adding sample 2 (metal scavenger) to each solutions, the solutions were left for a certain amount of time and then filtered.
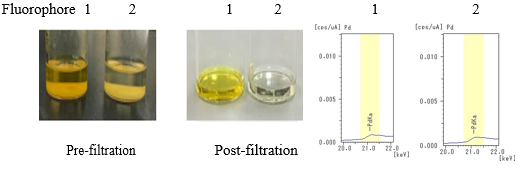
The separated powder after filtration

Pharmaceuticals Impurities Screening Method Package
Elements that can be analyzed with the pharmaceuticals impurities screening method package for XRF spectrometry are shown in Table below. Among the 24 elements listed in the ICH Q3D, a total of 12 elements can be analyzed.
Table: Elements That Can Be Analyzed with the Method Package
|
Classification |
Elements |
|
Class 1 Class 2A Class 2B |
Cd, Pb, As, Ni V, Co, Ni Ir, Pt, Ru, Rh, Pd |
Conclusion
Methods commonly used for analyzing inorganic impurities such as atomic absorption spectrophotometry, inductively coupled plasma (ICP) atomic emission spectroscopy, and ICP mass spectrometry require the wet digestion of solid and powder samples as pretreatment. However, by using XRF spectrometry, samples of all forms can be analyzed in their original form, such as a solution or powder, without pretreatment as long as the element to be analyzed is evenly dispersed in the sample.
This study shows that by using the pharmaceuticals impurities screening method package, an analysis in the order of mg/kg can be performed even when the integration time is substantially shortened to 300 s from the standard condition of 1,800 s. The package is effective in controlling the residual amount of catalysts in accordance with control standards and target accuracy levels.
Source: ETA
Others
- TECOTEC GROUP ATTENDED SHIMADZU’S SERVICE MANAGER MEETING IN 2022
- TECOTEC HANDED OVER EDX-7000 X-RAY FLOURESCENCE SPECTROMETER AT NIDEC CHAUN CHOUNG VIETNAM
- INSTALLATION OF CHIP PROCESSING SYSTEM – LANNER/ GERMANY
- TECOTEC completed installation of EDX-LE Energy dispersive X-ray Fluorescence spectrometer at DYT Vina
- TECOTEC DELIVERED AND INSTALLED THE 2ND X-RAY FLUORESCENCE SPECTROMETER - EDX-LE PLUS AT TABUCHI
- TECOTEC Group has handed over PDA-7000 Optical Emissions Spectrometers for Nihon Plast Vietnam
- Bowman XRF Coating Measurement System For Electroless Nickel Plating
- TECOTEC DELIVERED AND INSTALLED SMX-2000 SYSTEM TO NIDEC TECHNO MOTOR VIETNAM



