News
Analysis of Inorganic Additives in Resin by FTIR and EDX
Additives enhance the quality of products by improving the functionality, workability, and stability of the materials used in the product. They are utilized in a wide range of products, including electronics, foods, pharmaceuticals, cosmetics, plastics, etc., and play an important role by adding value to products. The approach taken in the analysis of additives is dependent on whether the additive is organic or inorganic.
Organic additives are typically identified by first extracting the additives using a suitable pretreatment procedure, and after chromatographic separation of the extracted components, a suitable analytical instrument is used for qualitative analysis. On the other hand, comprehensive identification of inorganic additive components is typically based on the results obtained using elemental analysis, infrared spectroscopy or morphologic observation, etc1). Here, we introduce examples of analysis to obtain useful information regarding some typical inorganic additives, in addition to an example of analysis of a resin containing an inorganic additive using FTIR and EDX.

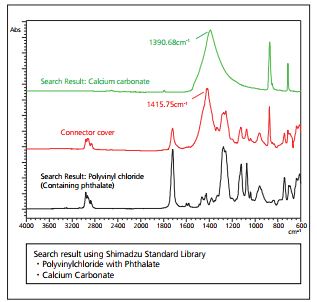
From the infrared spectra and search results of Fig. 6, the principal component of the connector cover was determined to be polyvinylchloride (PVC). Also, the peak in the vicinity of 1415 cm-1 in the infrared spectrum suggests the presence of calcium carbonate (CaCO3). However, a comparison of the connector cover peak at 1415 cm-1 with the peak at 1390 cm-1 of calcium carbonate alone indicates a peak position shift of 25 cm-1. Therefore, there is clearly insufficient basis to irrefutably conclude that calcium carbonate is included as an additive from the infrared spectrum alone and using EDX analysis to reconcile this discrepancy.
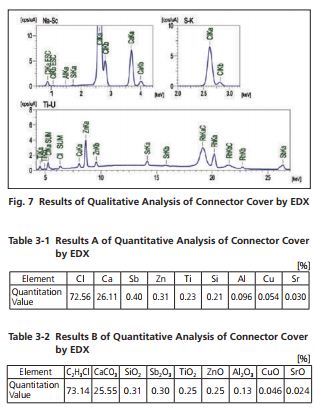
As clearly indicated in Table 3-1, chlorine (Cl) and calcium (Ca) are the principal constituent elements. This is consistent with the polyvinyl chloride results obtained by FTIR, supporting the presence of calcium carbonate. Table 3-2 shows the quantitative analytical results for specific compounds identified from the results of both FTIR and EDX. It should be noted that other detected elements are assumed to be oxides ). Thus, the combination of FTIR and EDX has provided sufficient evidence that calcium carbonate is present as an additive.
Source: ETA
Others
- TECOTEC GROUP ATTENDED SHIMADZU’S SERVICE MANAGER MEETING IN 2022
- TECOTEC HANDED OVER EDX-7000 X-RAY FLOURESCENCE SPECTROMETER AT NIDEC CHAUN CHOUNG VIETNAM
- INSTALLATION OF CHIP PROCESSING SYSTEM – LANNER/ GERMANY
- TECOTEC completed installation of EDX-LE Energy dispersive X-ray Fluorescence spectrometer at DYT Vina
- TECOTEC DELIVERED AND INSTALLED THE 2ND X-RAY FLUORESCENCE SPECTROMETER - EDX-LE PLUS AT TABUCHI
- TECOTEC Group has handed over PDA-7000 Optical Emissions Spectrometers for Nihon Plast Vietnam
- Bowman XRF Coating Measurement System For Electroless Nickel Plating
- TECOTEC DELIVERED AND INSTALLED SMX-2000 SYSTEM TO NIDEC TECHNO MOTOR VIETNAM



