News
ANALYZING NITROGEN IN STEEL BY SHIMADZU OPTICAL EMISSION SPECTROMETER PDA-7000
The spectral wavelength for nitrogen is 149.262nm, where the wavelength for iron, at 149.25nm, and the wavelength for chromium, at 149.298, are located nearby. The figure below shows a plot of the nitrogen spectrum, along with nearby iron and chromium spectra.
The first-order spectrum is affected by iron and chromium, because the iron and chromium spectra overlap with the nitrogen spectrum in the middle due to inadequate resolution. In contrast, using the second-order spectrum provides twice the resolution and eliminates the effects of iron and chromium on nitrogen. In addition, continuous spectra of silicon are also known to exist near the nitrogen spectrum. Their effect can approximately be cut in half as well, by using the second-order line.
- High Sensitivity Photomultiplier
Since the second-order spectral line for nitrogen at 149.2nm appears where it overlaps with the first-order
spectral line at 298.4nm, measuring the second-order spectral line also measures the interfering first-order spectral line. Therefore, high sensitivity can be achieved by using a special detector (photomultiplier) that provides lower measurement sensitivity than previous models for first-order spectral lines, but high measurement sensitivity for second-order spectral lines, as shown below.
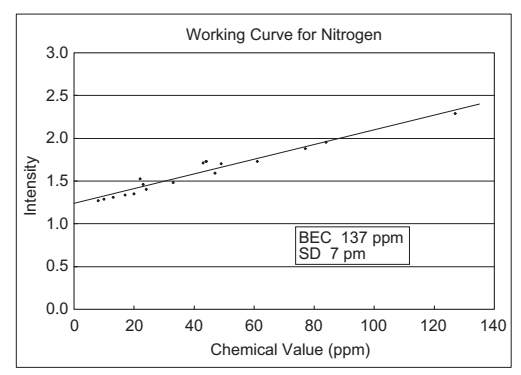
- High Energy Discharge
Discharge conditions were also considered and sensitivity was increased by using a 5 times higher energy spark discharge than for conventional discharge conditions.
Source: ETA
Others
- TECOTEC GROUP ATTENDED SHIMADZU’S SERVICE MANAGER MEETING IN 2022
- TECOTEC HANDED OVER EDX-7000 X-RAY FLOURESCENCE SPECTROMETER AT NIDEC CHAUN CHOUNG VIETNAM
- INSTALLATION OF CHIP PROCESSING SYSTEM – LANNER/ GERMANY
- TECOTEC completed installation of EDX-LE Energy dispersive X-ray Fluorescence spectrometer at DYT Vina
- TECOTEC DELIVERED AND INSTALLED THE 2ND X-RAY FLUORESCENCE SPECTROMETER - EDX-LE PLUS AT TABUCHI
- TECOTEC Group has handed over PDA-7000 Optical Emissions Spectrometers for Nihon Plast Vietnam
- Bowman XRF Coating Measurement System For Electroless Nickel Plating
- TECOTEC DELIVERED AND INSTALLED SMX-2000 SYSTEM TO NIDEC TECHNO MOTOR VIETNAM



