News
WHAT IS OPTICAL EMISSION SPECTROSCOPY (OES)?
Optical Emission Spectroscopy, or OES, is a well trusted and widely used analytical technique used to determine the elemental composition of a broad range of metals.

The type of samples which can be tested using OES include samples from the melt in primary and secondary metal production, and in the metals processing industries, tubes, bolts, rods, wires, plates and many more.
The part of the electromagnetic spectrum which is used by OES includes the visible spectrum and part of the ultraviolet spectrum. In terms of wavelength, that’s from 130 nanometers up to around 800 nanometers.
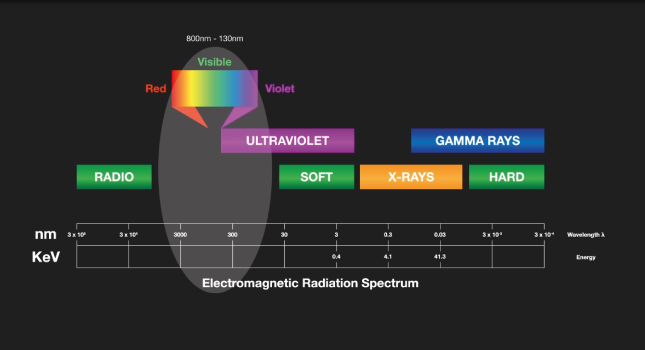
OES can analyze a wide range of elements from Lithium to Uranium in solid metal examples covering a wide concentration range, giving very high accuracy, high precision and low detection limits.
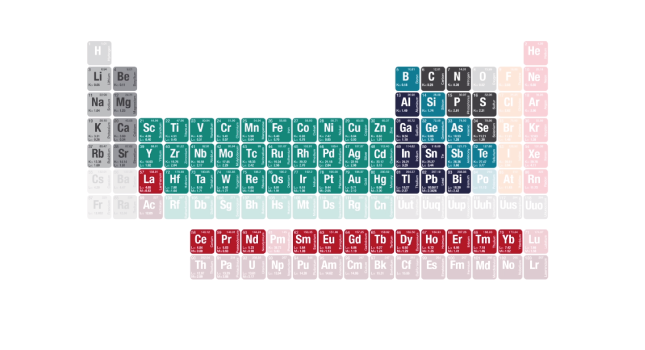
The elements and concentrations that OES analyzers can determine depend on the material being tested and the type of analyzer used.
How does Optical Emission Spectroscopy work?
All OES analyzers contain three major components, the first is an electrical source to excite atoms within a metallic sample so that they emit characteristic light, or optical emission, lines – requires a small part of the sample to be heated to thousands of degrees Celsius. This is done using an electrical high voltage source in the spectrometer via an electrode. The difference in electrical potential between the sample and electrode produces an electrical discharge, this discharge passes through the sample, heating and vaporizing the material at the surface and exciting the atoms of the material, which then emits the element-characteristic emission lines.
Two forms of electrical discharge can be generated, either an arc which is an on/off event similar to a lightning strike, or a spark – a series of multi-discharge events where the voltage of the electrode is switched on and off. These two modes of operation are used depending on the element measured and the accuracy required.
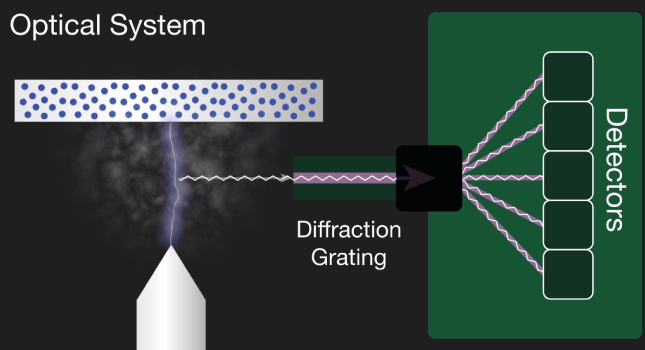
The second component is an optical system. The light, the multiple optical emission lines from the vaporized sample known as a plasma pass into the spectrometer. A diffraction grading in the spectrometer separates the incoming light into element-specific wavelengths and a corresponding detector measures the intensity of light for each wavelength. The intensity measured is proportional to the concentration offset element in the sample.
Compared to other analytical techniques, OES has many advantages: it’s fast and relatively easy to use, it measures a wide range of elements and concentrations in many different types of materials, including important elements such as carbon, sulfur, phosphorous, boron and nitrogen. It’s extremely accurate when measuring low levels of trace and tramp elements, and it’s fairly low-cost compared to other techniques.
For trace analysis of metals OES is the preferred method, OES is also currently the only method which can analyze carbon and nitrogen on site, out of the laboratory.
With more than 140 years of Shimadzu's accumulated experience in spectroscopy equipment, Shimadzu has supplied the world with the Optical Emission Spectrometer instruments that are indispensable for the quality control of metals. Shimadzu PDA-5000/7000/8000 and PDA-MF series, which combines the best of all these instruments.Shimadzu's unique time-resolution PDA photometry is installed as standard feature so as to provide powerful backup for quality control analysis, which is trusted by corporations in the world such as Honda, Yamaha, Hyundai…
Source: ETA
Others
- TECOTEC GROUP ATTENDED SHIMADZU’S SERVICE MANAGER MEETING IN 2022
- TECOTEC HANDED OVER EDX-7000 X-RAY FLOURESCENCE SPECTROMETER AT NIDEC CHAUN CHOUNG VIETNAM
- INSTALLATION OF CHIP PROCESSING SYSTEM – LANNER/ GERMANY
- TECOTEC completed installation of EDX-LE Energy dispersive X-ray Fluorescence spectrometer at DYT Vina
- TECOTEC DELIVERED AND INSTALLED THE 2ND X-RAY FLUORESCENCE SPECTROMETER - EDX-LE PLUS AT TABUCHI
- TECOTEC Group has handed over PDA-7000 Optical Emissions Spectrometers for Nihon Plast Vietnam
- Bowman XRF Coating Measurement System For Electroless Nickel Plating
- TECOTEC DELIVERED AND INSTALLED SMX-2000 SYSTEM TO NIDEC TECHNO MOTOR VIETNAM



