News
X-ray fluorescence spectroscopy method in Pharmaceuticals
With simple operation and rapid analysis of harmful elements, X-ray fluorescence spectroscopy (XRF) method is applied in not only Electricity but also Pharmaceutical industry.
In the pharmaceutical industry, the analysis of elemental impurities is necessary to ensure the safety of pharmaceuticals. In December 2014, the "Guideline for Elemental Impurities" (Q3D) was issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), consisting of representatives from Europe, the U.S. and Japan. In Japan, the "Guideline for Elemental Impurities in Drug Products" (PFSB/ELD Notification 0930 #4 from the Ministry of Health, Labour and Welfare) was issued, and will be applied to new drug products submitted for approval after April 1 2017. For 24 elements categorized in Class 1 to Class 3, residual quantities in pharmaceutical drug products must be controlled within permissible limits.
Although ICP-AES and ICP-MS are used for precise analysis of elemental impurities, X-ray fluorescence spectrometers can be used as an alternative analysis method. This is because they can quantitatively and qualitatively analyze a variety of elements nondestructively, and without chemical pretreatment, unlike ICP-AES and ICP-MS systems. The X-ray fluorescence spectrometry has been adopted as a general method of analysis in the U.S Pharmacopeia and the European Pharmacopoeia. (USP39<735>, Ph.Eur.2.2.37)
Element Classification (24 Elements)
Class 1: Very toxic. Highly toxic in all administration routes.
Class 2: Toxic, although it depends somewhat on the administration route.
Class 2A: Assessment is required in all cases.
Class 2AB: Assessment is required only when it is intentionally added in a process.
Class 3: Toxicity is low in oral administration. Assessment is required for other administration routes.
Detected Element Range and Elements Subject to the ICH Q3D Guideline
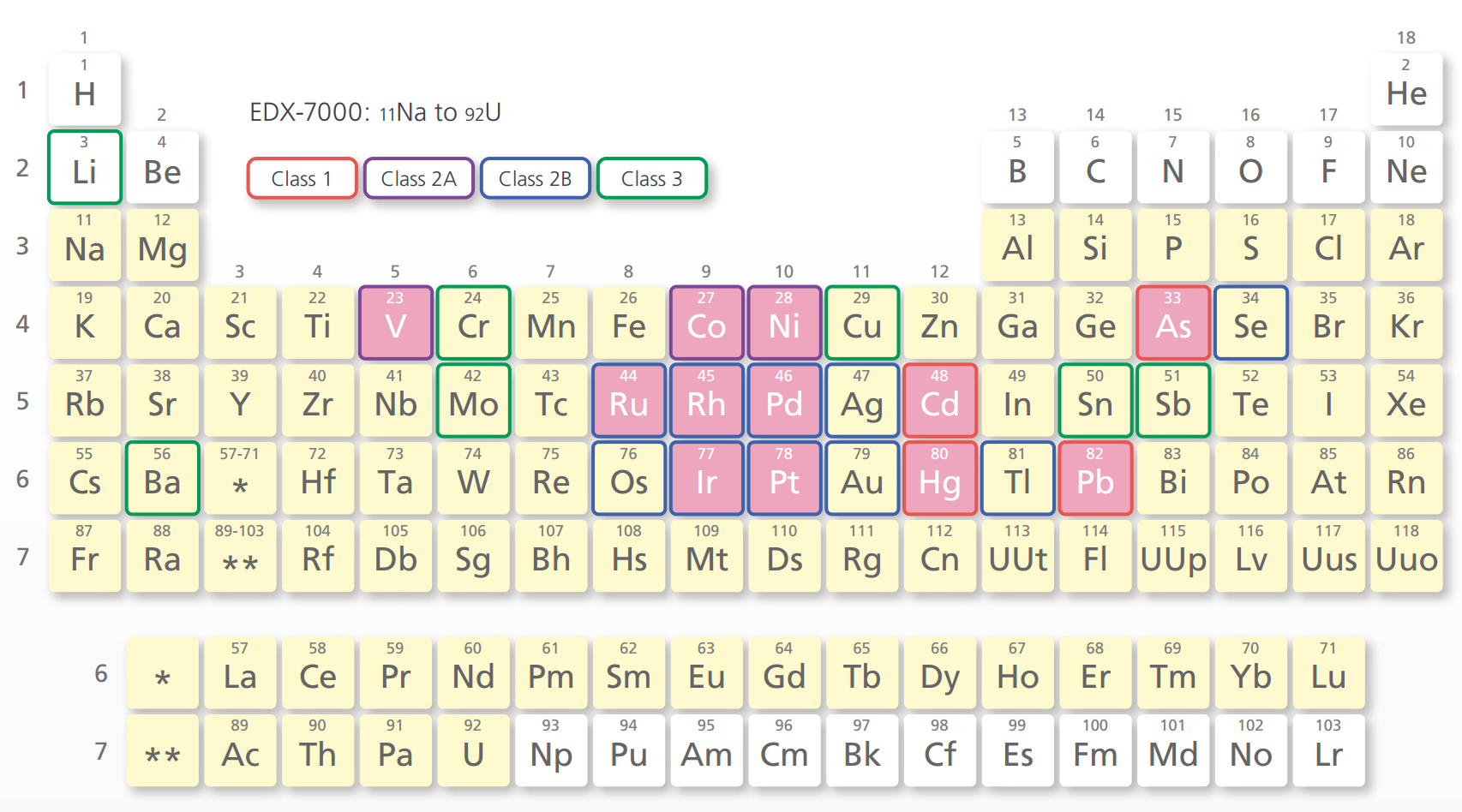
- An optional vacuum measurement unit or helium purge unit is required to measure light elements (15P and below) with the EDX-7000.
- The pink grid squares indicate elements that can be accommodated by the Pharmaceuticals Impurities Analysis Method Package (optional)
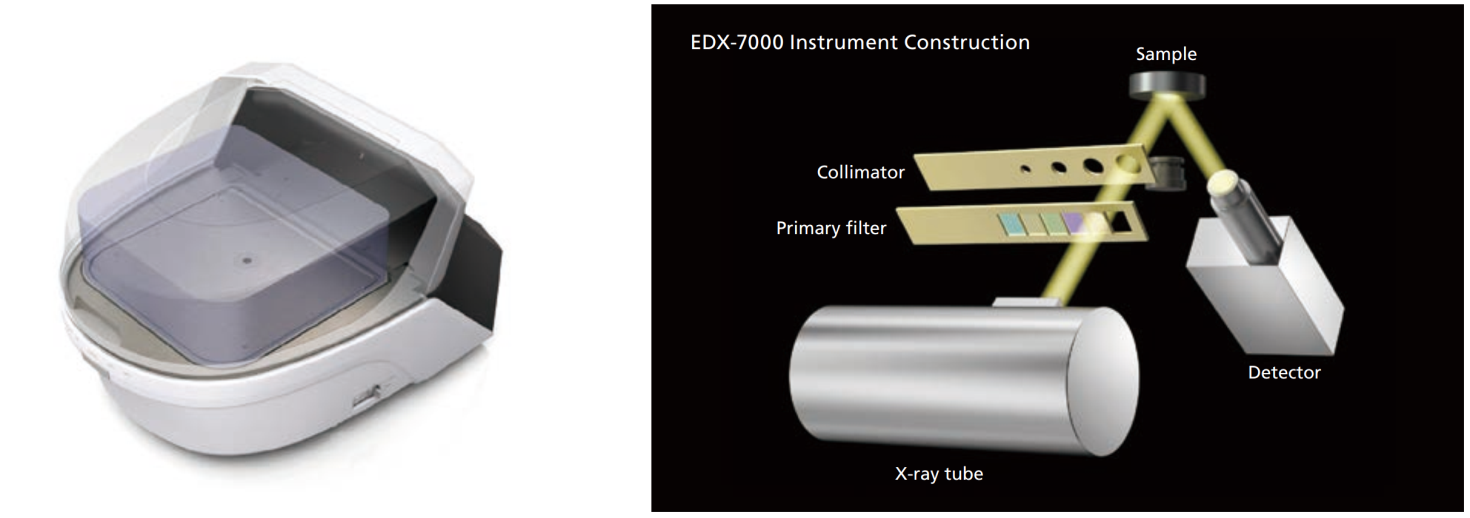
Instrument Construction
The optical system is a bottom exposure type in which both the X-ray tube and the detector are built into the bottom of the instrument. Samples simply need to be placed in the measurement area (the part with the hole) within the sample chamber. The instrument is so designed that the shutter on the X-ray tube will not open unless the sample chamber lid is completely closed, which prevents X-rays from leaking outside the instrument.
Positioning Powder and Liquid Samples
When analyzing powder and liquid samples that cannot be positioned as is in the measurement area, place the sample in a sample cell covered with a special film for X-ray fluorescence. Then start analysis by irradiating the sample with X-rays through the film.

Translation from source: http://www.shimadzu.com/an/elemental/edxrf/pharma_impurities/index.html
Source: ETA
Others
- TECOTEC GROUP ATTENDED SHIMADZU’S SERVICE MANAGER MEETING IN 2022
- TECOTEC HANDED OVER EDX-7000 X-RAY FLOURESCENCE SPECTROMETER AT NIDEC CHAUN CHOUNG VIETNAM
- INSTALLATION OF CHIP PROCESSING SYSTEM – LANNER/ GERMANY
- TECOTEC completed installation of EDX-LE Energy dispersive X-ray Fluorescence spectrometer at DYT Vina
- TECOTEC DELIVERED AND INSTALLED THE 2ND X-RAY FLUORESCENCE SPECTROMETER - EDX-LE PLUS AT TABUCHI
- TECOTEC Group has handed over PDA-7000 Optical Emissions Spectrometers for Nihon Plast Vietnam
- Bowman XRF Coating Measurement System For Electroless Nickel Plating
- TECOTEC DELIVERED AND INSTALLED SMX-2000 SYSTEM TO NIDEC TECHNO MOTOR VIETNAM



